Aliaxin
The complete intradermal dermal filler range containing ultrapure hyaluronic acid with the ability seamlessly integrate into the skin. A non-invasive, innovative treatment for natural contouring, lifting and hydration. Every area of the face requires a personalised treatment. IBSA has created four different formulations using different molecular weights with specific visco-elastic properties, offering a complete product line for the simultaneous treatment of 12 different facial areas.
Aliaxin® is an ultrapure hyaluronic acid filler which can be used to hydrate facial tissues as well as to revolumise, contour and lift facial features.
The Aliaxin® range of products is very versatile with an option to suit whatever purpose is required:
- Hydrating skin tissues.
- Tackling lines and wrinkles.
- Smoothing out creases including nose to mouth lines.
- Improving facial hollows e.g. tear troughs, around the eyes, cheeks.
- Facial contouring e.g. nose reshaping, cheek or chin enhancement, tackling asymmetry.
- Improving the texture of the skin with better elasticity and texture. Studies show skin remained 50% smoother after treatment.
- Producing a natural ‘lifting’ effect for non-surgical facelift or 8-point facelift procedures Aliaxin® also stimulates the body’s regeneration of collagen structures within the skin.
Hyaluronic acid fillers
for facial volume restoration and contouring
Hyaluronic acid (HA) is commonly used in aesthetic medicine, often mixed with lidocaine to reduce injection discomfort/pain. However, this approach still raises some concerns relating to the possible impairment of quality and efficacy, and possible allergic reactions. Aliaxin EV was effective in reducing nasolabial folds, giving a satisfying aesthetic performance with very well tolerated results, both with and without the addition of lidocaine. Aliaxin EV claims a high-volumizing capacity, indicated for use at the supraperiosteal layer and deep tissue, ideal for redefining facial contour, for correcting deficits following injuries, and for volume restoration. AGP is indicated for deep infiltrations, for the restoration of small volumes and to fill deep folds and wrinkles.

Figure1. Clinical photo series of three stages of IOH treatment. (A) Lateral Cheek; (B) Medial Cheek; (C) Palpebromalar groove; (D) Tear trough; (E) Result immediately after left side treated. Note the canthal tilt improvement.
Optimal lifting effect Aliaxin® Formulations
The Aliaxin® range offers a choice of 4 formulations which use different molecular weights and therefore offer different properties, providing the practitioner and patient with the perfect product depending on the treatment being undertaken:
Aliaxin GP
“Global performance”
- For treating common facial imperfections.
- Reshaping and re-volumising action.
- Correcting wrinkles, creases and folds.
- Ideal for nasolabial folds, nose, chin, lip augmentation and cheeks.
Aliaxin EV
“Essential volume”
- Suitable for contouring and reshaping.
- Restoring facial volume which depletes with ageing or weight loss.
- Optimal lifting effect.
Aliaxin SR
“Shape & Restore”
- Ideal for dermal remodelling.
- Replenishing lost volume.
- Providing natural-looking contours.
- Suits tear troughs /temples and other broad areas.
- Suitable for sensitive areas.
Aliaxin FL
“Fine lines”
- Recommended for lip enhancement.
- Hydrating properties.
- Ideal viscosity for restoring fullness.
- Define vermillion border of the lips.
- Specific treatments for fine lines.

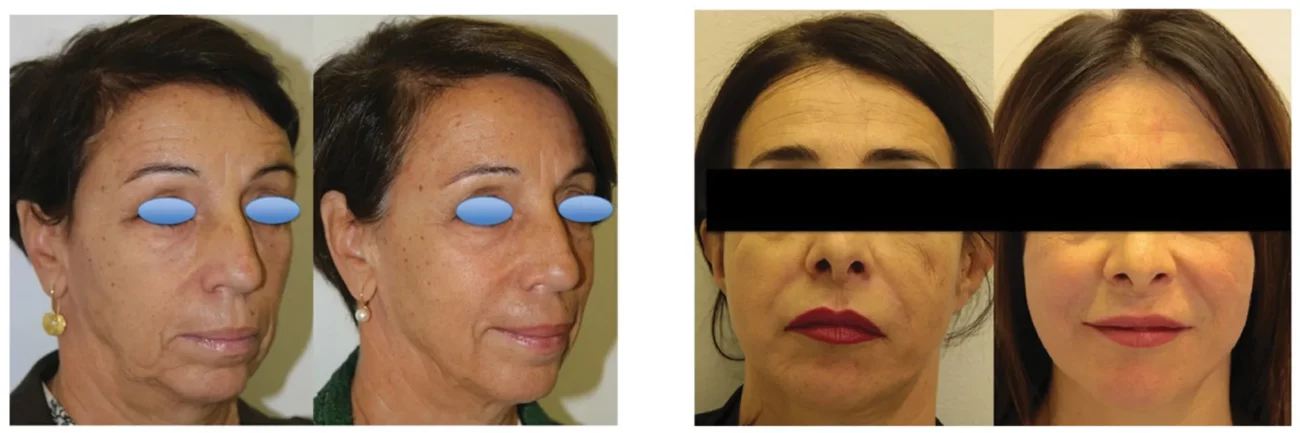
Figure 6. Representative pictures of two patients obtained before and 12 months after the last of three treatments performed at 3-month intervals. Patients provided written informed consent for the use of the images for scientific research.

Figure 7. Photographs of two patients before, post-immediate, 6 months and 18 months after treatment. Patient above treated with Aliaxin® LV. Patient below treated with Aliaxin® FL. Patients provided written informed consent for the use of the images for scientific research.
References
Ribé N. A technical approach for redefinition and volumization of lip area with hyaluronic acid: A case series. J Cosmet Dermatol. 2023;22(6):1739-1744. doi: 10.1111/jocd.15749.
Van Loghem J, Sattler S, Casabona G, Cotofana S, Fabi SG, Goldie K, Gout U, Kerscher M, Lim TS, de Sanctis Pecora C, Sattler G, Trindade de Almeida A, Wanitphakdeedecha R, Werschler P, Pavicic T. Consensus on the Use of Hyaluronic Acid Fillers from the Cohesive Polydensified Matrix Range: Best Practice in Specific Facial Indications. Clin Cosmet Investig Dermatol. 2021; 14:1175-1199. doi: 10.2147/CCID.S311017.
La Gatta A, Schiraldi C, Zaccaria G, Cassuto D. Hyaluronan Dermal Fillers: Efforts Towards a Wider Biophysical Characterization and the Correlation of the Biophysical Parameters to the Clinical Outcome. Clin Cosmet Investig Dermatol. 2020; 13:87-97. doi: 10.2147/CCID.S220227.
França Wanick FB, Almeida Issa MC, Luiz RR, Soares Filho PJ, Olej B. Skin Remodeling Using Hyaluronic Acid Filler Injections in Photo-Aged Faces. Dermatol Surg. 2016 Mar;42(3):352-9. doi: 10.1097/DSS.0000000000000659.
Annalisa La Gatta, Mario De Rosa, Maria Assunta Frezza, Claudia Catalano, Marisa Meloni, Chiara Schiraldi,Biophysical and biological characterization of a new line of hyaluronan-based dermal fillers: A scientific rationale to specific clinical. indications,Materials Science and Engineering,2016, 68Pages 565-572,https://doi.org/10.1016/j.msec.2016.06.008.