The aging process of the skin
Hyaluronic Acid (HA) is naturally found in our body and fulfills important functions such as maintaining moisture and volume in the skin. Environmental factors such as sun exposure and pollution, as well as the natural aging process have an influence on the depletion of soft tissue in the skin. As HA levels decline, skin becomes thinner, drier and less supple. This leads to the appearance of lines and wrinkles, and loss of volume which changes the harmony of the midface.The saypha® products are injectable gel implants made from a modified form of HA that can correct lines and wrinkles or restore volume.
Reasons to choose SAYPHA®
with saypha® VOLUME PLUS results at 1 month.
with saypha® FILLER at 36 weeks.
saypha® VOLUME to others.
View our before & after gallery of patient results
Individual results may vary. Talk to an Aesthetic Medical Professional to determine if saypha® is right for you.

This patient underwent mid face enhancement with 0.5 ml per side of saypha® VOLUME PLUS as well as treatment of the nasolabial folds with 0.3 ml per side of saypha® VOLUME.

This patient underwent mid face enhancement with 1 ml per side of saypha® VOLUME PLUS as well as treatment of the nasolabial folds with 0.5 ml per side of saypha® VOLUME.
Clinical Trials An open-label uncontrolled, multicenter study for the evaluation of the efficacy and safety of the dermal filler Princess® VOLUME2 in the treatment of nasolabial folds (PVN-1). PVN-1 study evaluated the effi cacy and safety of saypha® VOLUME in the treatment of moderate to severe nasolabial folds (NLFs) in 48 female and male subjects aged between 30 and 65 years.
over a period of nine months. The severity of NLFs was assessed by the investigator using the Modifi ed Fitzpatrick Wrinkle Scale (MFWS). A signifi cant improvement in MFWS and thus clinical effi cacy was demonstrated in the study. After 9 months more than half of the subjects still showed a signifi cant improvement, 85.1% demonstrated 1 grade improvement in both NLFs (fi gure 1). For the majority of subjects, the NLF appearance based on Global Aesthetic Improvement Scale (GAIS), as judged by the investigator was ‘much improved’ at all post-treatment visits (fi gure 2), with favorable aesthetic outcome reported for 97.9% of subjects at the study end.
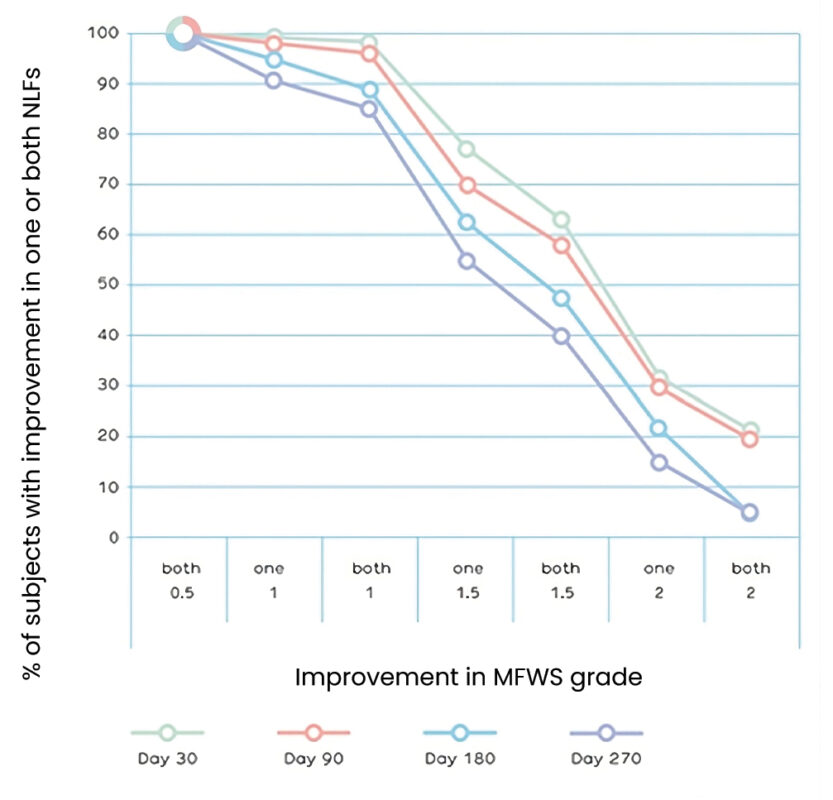
Figure 1. Percentage of subjects with an improvement in MFWS in one or both nasolabial folds.
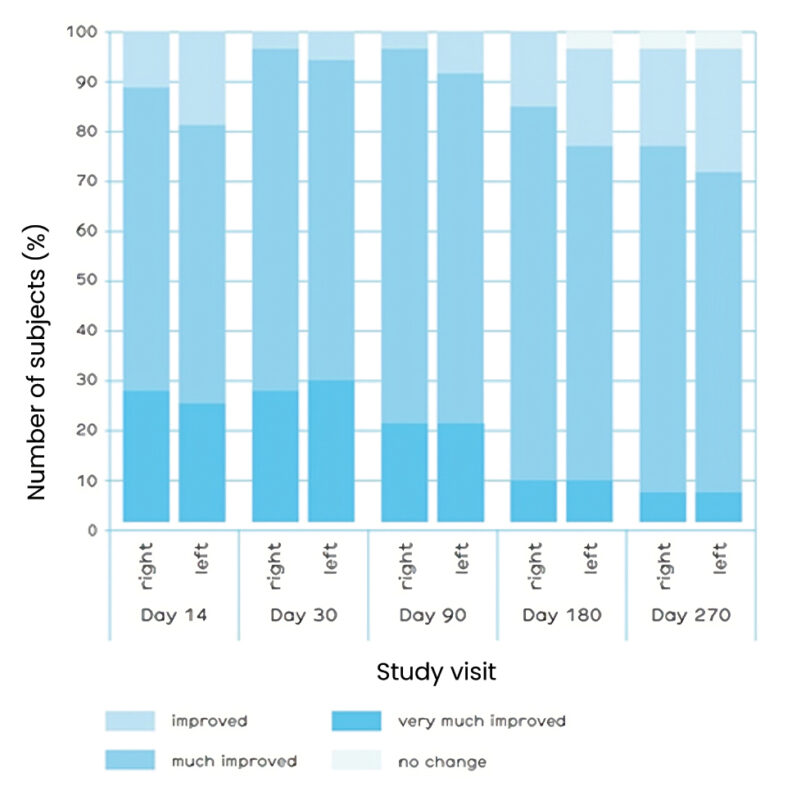
Figure 2. Global Aesthetic Improvement Scale assessments at each study visit. Designations right and left are corresponding to right and left nasolabial folds.
The majority of subjects (93.6%)
saypha® VOLUME was well tolerated in this investigation, with most adverse events (AEs) being injection site reactions of mild to moderate severity.
A prospective, split-face, randomized, evaluator and subject-blinded, multicenter, noninferiority study for the evaluation of safety and effectiveness of Princess® VOLUME for the correction of moderate to severe nasolabial folds in Chinese subjects. This study evaluated the efficacy and safety of saypha® VOLUME for the treatment of NLFs in 120 male and female Chinese subjects, with follow-up visits after one year in a split-face design (saypha® VOLUME in one NLF and Restylane® in the other NLF). The validated Wrinkle Severity Rating Scale (WSRS) and the GAIS were used to evaluate the efficacy.
The primary efficacy endpoint of the trial was the rate of improvement (≥1 grade) in the severity of NLFs at week 24 (assessed by an Independent Review Committee [IRC] using the 5 point WSRS and based on photographs – figure 3). saypha® VOLUME demonstrated statistical non-inferiority to Restylane®. The mean GAIS scores assessed by IRC and by subjects were rated better for saypha® VOLUME at all-time points tested, consistent with the reported greater reduction in severity of NLFs treated with saypha® VOLUME (figure 4). The rates of occurrence of AEs were comparable for both products and consistent with the literature, with most of the AEs lessened or disappeared within 2 weeks after injection.
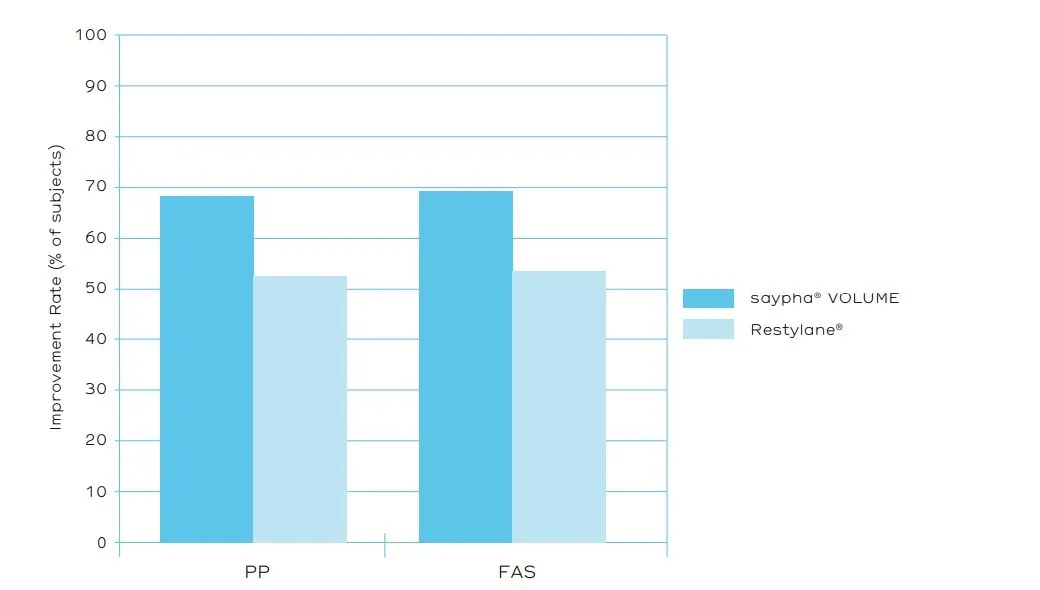
Figure 3. WSRS improvement rates assessed by the Independent Review Committee at 24 Weeks (PP = per-protocol; FAS = full analysis set).
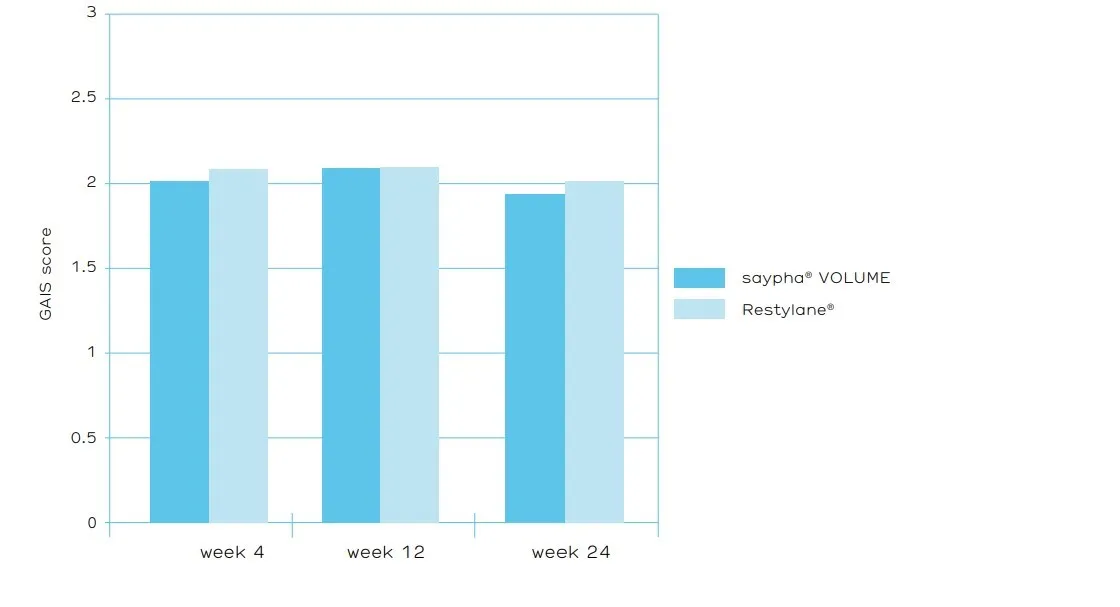
Figure 4. Assessment of the Global Aesthetic Improvement Scale (GAIS) score by the Independent Review Committee over time using Full Analysis Set (FAS) population.
A prospective, open label, multicenter, post-market clinical follow-up study of the Princess® VOLUME2 performance and safety for correction of facial lipoatrophy, morphological asymmetry of the face, or debilitating scars (CPH-401-201260; FLASH 2).
This clinical investigation evaluated the performance and safety of saypha® VOLUME for the correction of facial lipoatrophy, morphological asymmetry, or debilitating scars in 56 male and female patients (55 Caucasian, 1 black; 18–90 years of age) for 36 weeks. Overall, the successful treatment outcome rate was very high and showed only marginal differences between indications. The rate of AEs was very low, and all resolved without sequelae. The investigation showed the treatment with saypha® VOLUME to be safe, well tolerated and efficacious in the treatment of facial lipoatrophy, morphological asymmetry and debilitating scars.
Conclusions
Dedicated clinical studies with saypha® VOLUME clearly demonstrated effectiveness in correction of nasolabial folds, as well as high success rate in treatment of facial lipoatrophy, morphological asymmetry of the face or debilitating scars in male and female subjects aged between 18 and 90 years, confi rming the effi cacy, patient satisfaction and safety of use.
The saypha® portfolio of Hyaluronic Acid Dermal Fillers are specially designed to meet specific patient needs:
- Saypha® FILLER is used to correct light to moderate nasolabial folds.
- Saypha® VOLUME is used to correct deep wrinkles in the nasolabial folds.
- Saypha® VOLUME PLUS is used to correct moderate to severe midface volume deficit.
References
1. Kopera D., Palatin M., Bartsch R., Bartsch K., O’Rourke M., Hoeller S., Baumgartner R. R. & Prinz M. 2015. An open-label uncontrolled, multicenter study for the evaluation of the efficacy and safety of the dermal filler Princess® VOLUME in the treatment of nasolabial folds.
2. Biomed Res Int, 2015, 195328 4. Dai X., Li L., Peterson W., Baumgartner R. R., Huang J., Baer-Zwick A., Hoeller S., Ivezic-Schoenfeld Z. & Prinz M. 2018. Safety and effectiveness of hyaluronic acid dermal filler in correction of moderate-to-severe nasolabial folds in Chinese subjects. Clinical, Cosmetic and Investigational Dermatology 2019:12 57–62 5
3. Croma data on file The medical practitioner confirms having informed the patient of a likely risk associated with the use of the product consult the instructions of use.